The management of cough symptoms represents one of the most common therapeutic challenges in both clinical practice and self-care medicine. Understanding the fundamental differences between antitussive medications and expectorants requires a comprehensive appreciation of cough physiology, the various pathological mechanisms underlying different cough types, and the specific pharmacological approaches designed to address each distinct clinical presentation. The complexity of cough as a symptom stems from its dual nature as both a protective physiological mechanism and a potentially troublesome pathological condition that significantly impacts quality of life and daily functioning.
Cough serves as a critical defense mechanism for the respiratory system, functioning to clear foreign materials, excessive secretions, and irritants from the airways while protecting the lower respiratory tract from aspiration and infection. However, when cough becomes excessive, persistent, or unproductive, it transforms from a beneficial reflex into a source of discomfort, sleep disruption, social embarrassment, and potential complications including exhaustion, musculoskeletal pain, and even rib fractures in severe cases.
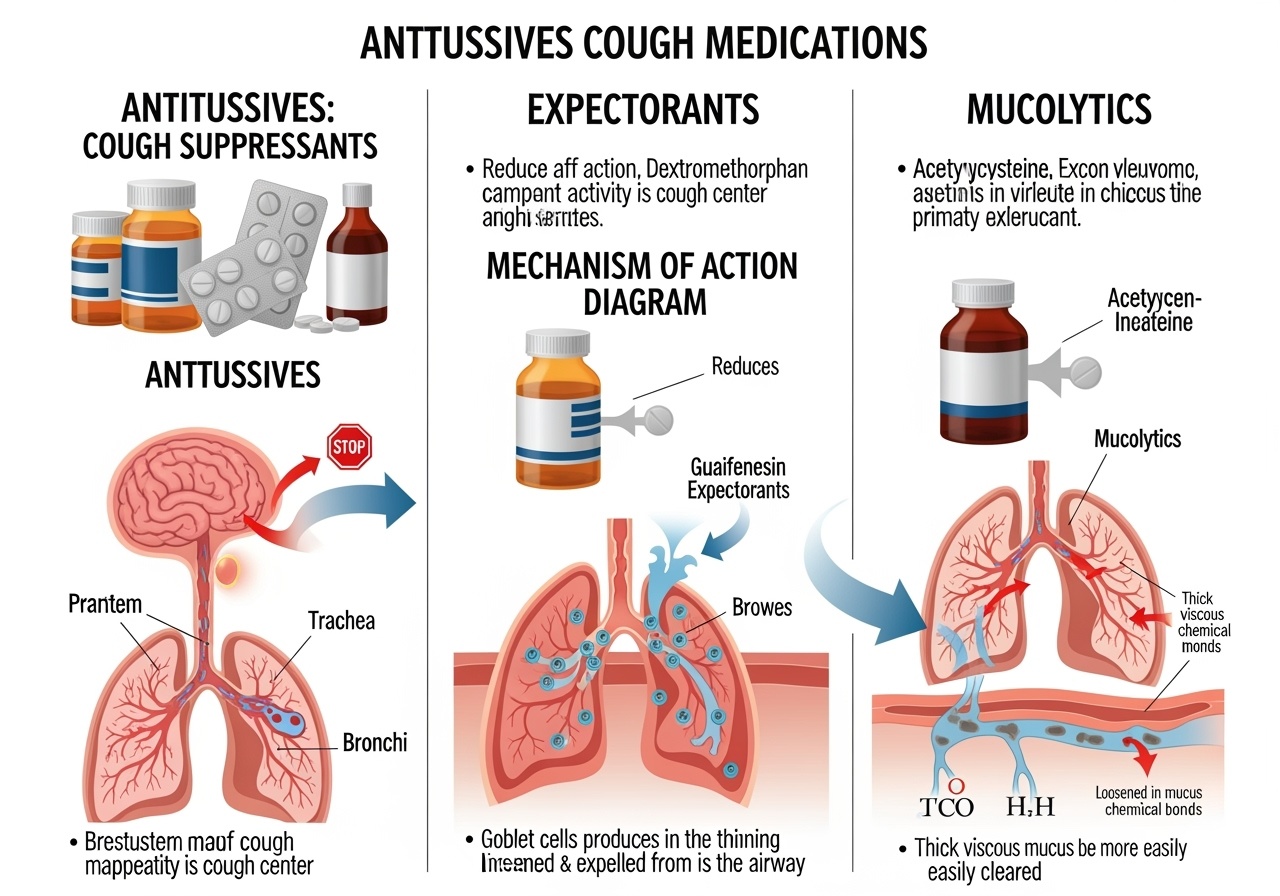
The pharmaceutical approach to cough management has evolved significantly over decades of clinical research and practice, resulting in distinct categories of medications designed to address different aspects of the cough reflex and its underlying pathophysiology. Antitussive agents work by suppressing the cough reflex itself, providing relief from persistent, non-productive coughing that serves no beneficial purpose. Expectorant medications take a fundamentally different approach by facilitating the clearance of respiratory secretions, making productive coughs more effective and less burdensome.
The decision between these therapeutic approaches requires careful consideration of multiple factors including the nature of the cough, the presence or absence of sputum production, underlying respiratory conditions, patient age and comorbidities, potential drug interactions, and individual response patterns. This comprehensive analysis provides healthcare providers and patients with the detailed information necessary to make informed decisions about cough medication selection and optimize therapeutic outcomes while minimizing adverse effects and complications.
Understanding the Physiology of Cough and Its Therapeutic Implications
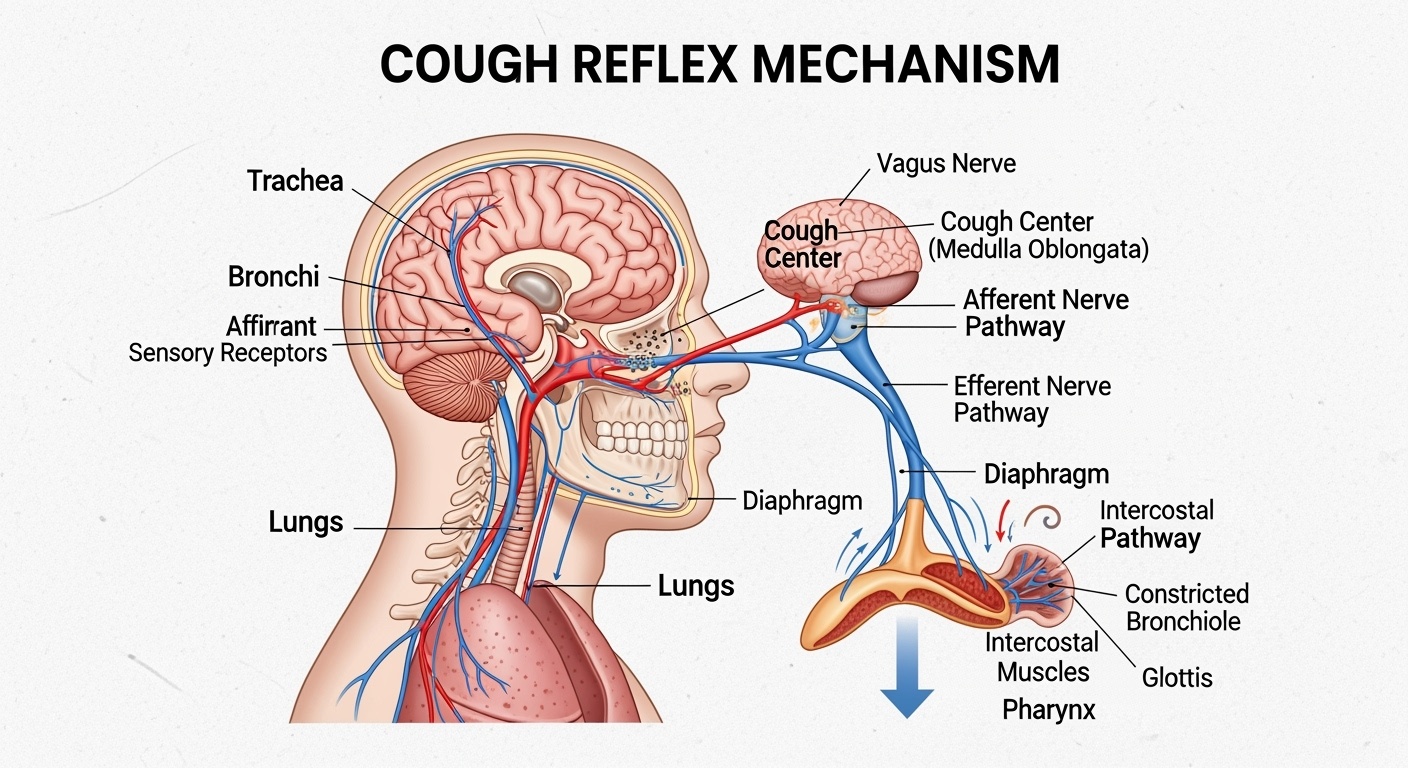
The cough reflex represents a sophisticated neurological pathway that involves multiple anatomical structures and physiological processes working in coordinated fashion to protect respiratory function. The initiation of cough begins with the stimulation of specialized sensory receptors located throughout the respiratory tract, including the upper airways, trachea, bronchi, and even the pleural surfaces. These receptors, primarily consisting of rapidly adapting mechanoreceptors and chemically sensitive nerve endings, respond to various stimuli including mechanical irritation, chemical irritants, inflammatory mediators, and changes in airway wall tension.
When these receptors detect potentially harmful stimuli, they generate afferent signals that travel via the vagus nerve and other cranial nerves to the cough center located in the medulla oblongata of the brainstem. This cough center functions as an integration point where incoming sensory information is processed and evaluated to determine whether a cough response is warranted. The complexity of this central processing allows for modulation of the cough response based on higher cortical input, emotional state, and conscious control, explaining why cough can sometimes be voluntarily suppressed or, conversely, triggered by psychological factors.
Once the cough center determines that a cough response is appropriate, it initiates a complex motor sequence involving multiple muscle groups and respiratory structures. The cough sequence consists of three distinct phases that must occur in precise coordination to achieve effective airway clearance. The inspiratory phase involves deep inspiration that increases lung volume and positions the respiratory system for maximum expulsive force. The compressive phase follows, during which the glottis closes while respiratory muscles contract against the closed airway, generating high intrathoracic pressure. Finally, the expulsive phase occurs when the glottis suddenly opens, creating rapid airflow that can reach velocities exceeding 500 miles per hour, effectively clearing materials from the respiratory tract.
Understanding this physiological framework is crucial for appreciating how different classes of cough medications exert their therapeutic effects. Antitussive medications primarily target the central nervous system components of the cough reflex, either by raising the threshold for cough center activation or by interfering with the neural transmission pathways involved in cough initiation. Expectorant medications, in contrast, focus on modifying the physical properties of respiratory secretions and enhancing the mechanical effectiveness of the cough mechanism itself.
The pathophysiology underlying different types of coughs also influences medication selection and effectiveness. Dry, non-productive coughs often result from upper respiratory tract irritation, allergic responses, or hypersensitive cough reflexes that trigger coughing in response to minimal stimuli. These coughs typically do not serve a beneficial purpose and may be appropriately managed with antitussive therapy. Productive coughs, however, usually indicate the presence of respiratory secretions that need to be cleared from the airways, making expectorant therapy more appropriate to enhance the natural clearance mechanisms.
The temporal patterns of cough also provide important diagnostic and therapeutic information. Acute coughs lasting less than three weeks are typically associated with viral respiratory infections and may benefit from symptomatic treatment with either antitussives or expectorants depending on their characteristics. Subacute coughs persisting for three to eight weeks often represent the resolution phase of respiratory infections and may require different therapeutic approaches. Chronic coughs lasting more than eight weeks usually indicate underlying pathological conditions that require specific diagnosis and targeted treatment beyond symptomatic cough suppression.
Antitussive Medications: Mechanisms, Applications, and Clinical Considerations
Antitussive medications represent a diverse class of pharmaceutical agents united by their common ability to suppress the cough reflex through various mechanisms of action. The primary goal of antitussive therapy is to reduce the frequency, intensity, and duration of coughing episodes, particularly when the cough is non-productive and provides no therapeutic benefit to the patient. Understanding the different categories of antitussive medications and their specific mechanisms of action is essential for optimal therapeutic selection and patient counseling.
Centrally acting antitussives represent the most commonly used class of cough suppressants, working by directly affecting the cough center in the medulla oblongata. Dextromethorphan stands as the most widely available and frequently used over-the-counter antitussive agent, functioning as an NMDA receptor antagonist that raises the threshold for cough center activation. This mechanism differs significantly from that of opioid antitussives, making dextromethorphan less likely to cause respiratory depression or addiction potential while maintaining significant cough suppression efficacy.
The pharmacokinetics of dextromethorphan involve hepatic metabolism through the cytochrome P450 system, particularly CYP2D6, which shows significant genetic polymorphism affecting individual response patterns. Poor metabolizers of CYP2D6 may experience enhanced and prolonged effects from standard doses, while ultra-rapid metabolizers may require higher doses for therapeutic efficacy. This genetic variability explains the considerable individual differences observed in dextromethorphan effectiveness and emphasizes the importance of individualized dosing approaches.
Dextromethorphan is typically administered in doses ranging from 15 to 30 milligrams every four to six hours for adults, with maximum daily doses generally not exceeding 120 milligrams. Pediatric dosing requires careful attention to age-appropriate formulations and weight-based calculations, with many experts recommending against use in children under four years of age due to safety concerns and limited efficacy data. Extended-release formulations provide prolonged cough suppression with less frequent dosing, improving patient compliance and maintaining consistent therapeutic levels.
Codeine and other opioid antitussives represent a more potent class of centrally acting cough suppressants that work through activation of opioid receptors in the cough center. While highly effective for cough suppression, these medications carry significant risks including respiratory depression, sedation, constipation, and addiction potential. The use of codeine-containing cough preparations has become increasingly restricted due to safety concerns, particularly in pediatric populations where several fatalities have been reported.
The mechanism of opioid antitussives involves binding to mu-opioid receptors in the central nervous system, not only affecting the cough center but also producing generalized central nervous system depression. This broad activity profile explains both the high efficacy of opioid antitussives and their significant side effect potential. Respiratory depression represents the most serious concern, particularly in patients with underlying respiratory compromise, sleep apnea, or when combined with other central nervous system depressants.
Peripherally acting antitussives work through different mechanisms that target the sensory components of the cough reflex pathway. These medications may act as local anesthetics on respiratory tract sensory receptors, modify neurotransmitter release at peripheral nerve endings, or alter the sensitivity of mechanoreceptors and chemoreceptors involved in cough initiation. While generally associated with fewer systemic side effects than centrally acting agents, peripherally acting antitussives may have more limited efficacy for severe cough symptoms.
Benzonatate represents a unique peripherally acting antitussive that functions as a local anesthetic on respiratory tract sensory receptors. Available in 100 and 200-milligram capsules, benzonatate is typically administered three times daily and can provide cough suppression for up to eight hours per dose. The medication must be swallowed whole without chewing or crushing, as release of the contents in the mouth can cause severe local anesthesia and choking hazards.
The selection of appropriate antitussive therapy requires careful consideration of multiple patient factors including age, underlying medical conditions, concurrent medications, and the specific characteristics of the cough being treated. Dry, hacking coughs that interfere with sleep or daily activities represent ideal candidates for antitussive therapy, while productive coughs with significant sputum production may be better managed with alternative approaches.
Contraindications to antitussive use include productive coughs where suppression might impair beneficial airway clearance, patients with chronic obstructive pulmonary disease or other conditions where cough suppression could lead to secretion retention, and individuals with known hypersensitivity to specific antitussive agents. Special caution is required in patients with respiratory compromise, as excessive cough suppression could potentially worsen ventilation and gas exchange.
Expectorant Medications: Enhancing Natural Clearance Mechanisms
Expectorant medications represent a fundamentally different approach to cough management, focusing on enhancing the effectiveness of productive coughs rather than suppressing the cough reflex itself. The primary mechanism of expectorant action involves modification of respiratory secretion properties to make them less viscous and easier to expectorate, thereby improving the natural clearance mechanisms of the respiratory tract and reducing the effort required for effective coughing.
Guaifenesin stands as the most widely used and extensively studied expectorant medication, with a long history of clinical use and generally favorable safety profile. The precise mechanism by which guaifenesin exerts its expectorant effects remains somewhat controversial, with proposed mechanisms including direct stimulation of respiratory tract secretory glands, modification of mucus viscoelastic properties, and enhancement of ciliary function. Regardless of the exact mechanism, clinical studies have demonstrated that guaifenesin can effectively reduce sputum viscosity and improve cough productivity in patients with respiratory conditions characterized by thick, tenacious secretions.
The pharmacokinetics of guaifenesin involve rapid absorption from the gastrointestinal tract with peak plasma concentrations achieved within one to two hours after oral administration. The medication undergoes hepatic metabolism and renal elimination with a relatively short half-life of approximately one hour, necessitating frequent dosing for sustained therapeutic effects. Standard immediate-release formulations are typically administered every four hours, while extended-release formulations provide twelve-hour duration of action with twice-daily dosing.
Adult dosing of guaifenesin typically ranges from 200 to 400 milligrams every four hours for immediate-release formulations, with maximum daily doses generally not exceeding 2400 milligrams. Extended-release formulations are usually dosed at 600 to 1200 milligrams every twelve hours. Pediatric dosing requires age-appropriate calculations and formulations, with liquid preparations commonly used for younger children who cannot safely swallow tablets or capsules.
The therapeutic benefits of guaifenesin are most apparent in patients with productive coughs associated with thick, difficult-to-expectorate secretions. Conditions such as acute bronchitis, chronic bronchitis, and other respiratory tract infections often involve production of viscous sputum that can be challenging to clear effectively. By reducing sputum viscosity and improving its flow properties, guaifenesin can make productive coughs more effective and less exhausting for patients.
The role of adequate hydration in expectorant therapy cannot be overstated, as the effectiveness of these medications is significantly enhanced when patients maintain proper fluid intake. Dehydration can lead to increased secretion viscosity that counteracts the beneficial effects of expectorant medications, while adequate hydration supports the natural thinning of respiratory secretions and enhances drug effectiveness. Patients should be counseled to increase their fluid intake when using expectorant medications, unless contraindicated by underlying medical conditions.
Combination products containing both expectorant and other active ingredients are widely available and may provide broader symptom relief for patients with complex respiratory conditions. However, these combination products also increase the risk of adverse effects and drug interactions, requiring careful evaluation of individual patient needs and risk factors. The principle of using the simplest effective therapy suggests that single-ingredient expectorant products may be preferable when cough is the primary symptom requiring treatment.
The assessment of expectorant effectiveness requires patient education about realistic expectations and appropriate outcome measures. Unlike antitussive medications that reduce cough frequency, expectorants may initially increase cough frequency as respiratory secretions become easier to mobilize and expectorate. Patients should be counseled that this increased productivity represents a therapeutic benefit rather than treatment failure, and that persistence with therapy for several days may be necessary to achieve optimal results.
Contraindications to expectorant use are relatively limited but include known hypersensitivity to guaifenesin or other expectorant agents. Caution is warranted in patients with severe kidney disease due to the renal elimination pathway of guaifenesin, and in patients with phenylketonuria when using sugar-free formulations containing aspartame. The generally favorable safety profile of expectorant medications makes them suitable for use in most patient populations, including pregnant and breastfeeding women when clinically indicated.
Mucolytic Agents: Advanced Secretion Management
Mucolytic medications represent a specialized category of respiratory therapeutics that work through direct biochemical modification of mucus structure and composition. Unlike expectorants that primarily affect secretion production and flow properties, mucolytics achieve their therapeutic effects by breaking down the molecular bonds within existing mucus, particularly the disulfide bridges that contribute to mucus viscosity and adhesiveness. This direct action on mucus structure makes mucolytics particularly valuable for patients with conditions characterized by abnormally thick, tenacious secretions that are resistant to conventional expectorant therapy.
Acetylcysteine represents the most well-established mucolytic agent, with extensive clinical experience in both oral and inhalational formulations. The medication functions as a reducing agent that cleaves disulfide bonds within mucin glycoproteins, the primary structural components responsible for mucus viscosity. This biochemical action results in dramatic reduction of mucus viscosity and improved clearance from the respiratory tract, making acetylcysteine particularly valuable for patients with cystic fibrosis, chronic obstructive pulmonary disease, and other conditions characterized by abnormal mucus production.
The clinical applications of acetylcysteine extend beyond its mucolytic properties to include antioxidant effects and potential anti-inflammatory actions. As a precursor to glutathione, acetylcysteine can help restore cellular antioxidant capacity and may provide protective effects against oxidative damage in the respiratory tract. These additional properties make acetylcysteine particularly valuable for patients with chronic respiratory conditions where oxidative stress contributes to ongoing tissue damage and inflammation.
Oral acetylcysteine is typically administered in doses ranging from 200 to 600 milligrams two to three times daily, depending on the severity of symptoms and patient response. The medication has a characteristic sulfur odor and taste that some patients find objectionable, potentially affecting compliance with long-term therapy. Effervescent tablet formulations may improve palatability and dissolution characteristics compared to standard tablets or capsules.
Inhalational acetylcysteine provides direct delivery to the respiratory tract and may achieve higher local concentrations than oral therapy. However, inhalational administration can potentially trigger bronchospasm in susceptible patients, particularly those with asthma or reactive airway disease. Pre-treatment with bronchodilators may be necessary when using inhalational mucolytics in patients with a history of bronchial hyperreactivity.
Dornase alfa represents a newer class of mucolytic agent that works through enzymatic degradation of extracellular DNA present in purulent respiratory secretions. This recombinant human DNase enzyme is particularly effective in conditions where neutrophilic inflammation leads to release of significant amounts of DNA into respiratory secretions, as occurs in cystic fibrosis and severe bacterial respiratory infections. The high cost and specialized administration requirements of dornase alfa limit its use to specific clinical situations where conventional mucolytics prove inadequate.
The clinical effectiveness of mucolytic therapy requires patient selection based on the characteristics of respiratory secretions and underlying pathophysiology. Patients with thin, watery secretions are unlikely to benefit significantly from mucolytic therapy, while those with thick, purulent, or visibly tenacious sputum may experience substantial improvement. The assessment of secretion characteristics should include consideration of color, consistency, volume, and ease of expectoration to guide appropriate therapeutic selection.
Adverse effects of mucolytic therapy are generally mild but can include gastrointestinal upset, nausea, and allergic reactions in susceptible individuals. The sulfur-containing nature of acetylcysteine can interact with certain metals and should be avoided in patients with active peptic ulcer disease due to potential irritant effects. Drug interactions are relatively uncommon but may include reduced absorption of certain antibiotics when administered concurrently with oral mucolytics.
Pediatric Considerations in Cough Medicine Selection
The management of cough symptoms in pediatric populations requires specialized knowledge of developmental physiology, age-specific safety considerations, and unique therapeutic challenges that distinguish pediatric practice from adult medicine. Children’s respiratory systems differ significantly from adults in terms of anatomy, physiology, and response to therapeutic interventions, necessitating careful consideration of these factors when selecting and dosing cough medications.
The anatomical differences in pediatric respiratory systems include smaller airway caliber, different chest wall mechanics, and immature respiratory muscle development that can affect both the pathophysiology of cough and the response to therapeutic interventions. The relatively smaller diameter of pediatric airways means that even minor degrees of mucosal swelling or increased secretions can cause proportionally greater obstruction and respiratory compromise compared to adults. This anatomical vulnerability requires particular caution when using medications that might affect respiratory function or airway patency.
Developmental differences in hepatic and renal function significantly impact drug metabolism and elimination in pediatric patients, requiring age-specific dosing adjustments and careful monitoring for adverse effects. The cytochrome P450 enzyme systems responsible for metabolizing many cough medications do not reach adult levels of activity until several years of age, potentially leading to prolonged drug effects and increased risk of toxicity in young children. Similarly, renal elimination pathways may be immature in infants and toddlers, affecting the clearance of medications that rely on kidney function for elimination.
The safety profile of cough medications in pediatric populations has received increased scrutiny following reports of serious adverse events and deaths associated with over-the-counter cough and cold medications in young children. Regulatory agencies have issued warnings and recommendations against the use of these medications in children under specific ages, with many experts recommending against use in children under four years of age due to limited efficacy data and documented safety concerns.
Dextromethorphan use in children requires particular attention to proper dosing and potential adverse effects. While generally considered safer than opioid antitussives, dextromethorphan can still cause significant central nervous system effects in children, including sedation, confusion, and in severe cases, respiratory depression. The wide variation in individual sensitivity to dextromethorphan, partially related to genetic polymorphisms in drug metabolism, makes it difficult to predict individual response patterns and emphasizes the importance of conservative dosing approaches.
The use of codeine-containing medications in pediatric populations has become increasingly restricted due to documented cases of severe respiratory depression and death, particularly in children who are ultra-rapid metabolizers of the CYP2D6 enzyme system. These individuals convert codeine to morphine at accelerated rates, leading to potentially toxic concentrations of the active metabolite. Current recommendations strongly discourage the use of codeine in children under twelve years of age and advise extreme caution in adolescents.
Expectorant medications generally have more favorable safety profiles in pediatric populations compared to antitussives, but still require appropriate dosing and monitoring. Guaifenesin is considered relatively safe for children when used at appropriate doses, but the effectiveness of expectorant therapy in pediatric patients may be limited by their inability to effectively expectorate mobilized secretions. Young children may require additional supportive measures such as chest physiotherapy or positioning to optimize secretion clearance.
Non-pharmacological approaches to cough management often represent the most appropriate first-line therapy for pediatric patients, particularly those under four years of age. These approaches include adequate hydration, humidification of inspired air, elevation of the head during sleep, and removal of environmental irritants. Honey has shown efficacy for cough suppression in children over one year of age and may provide a safer alternative to pharmaceutical antitussives for mild symptoms.
The assessment of cough severity and treatment response in pediatric patients requires age-appropriate evaluation methods and consideration of developmental factors that may affect symptom reporting. Young children may be unable to accurately describe their symptoms or cooperate with traditional assessment methods, requiring reliance on parental observations and objective measures such as sleep disruption, feeding difficulties, or activity limitations.
Drug Interactions and Safety Considerations
The complex pharmacology of cough medications creates numerous potential opportunities for clinically significant drug interactions that can affect both the efficacy and safety of therapeutic regimens. Understanding these interaction patterns is essential for healthcare providers and patients to optimize therapeutic outcomes while minimizing the risk of adverse effects or treatment failures.
Dextromethorphan interactions primarily involve the cytochrome P450 enzyme system, particularly CYP2D6, which is responsible for the majority of dextromethorphan metabolism. Medications that inhibit CYP2D6 activity can significantly increase dextromethorphan plasma concentrations and prolong its effects, potentially leading to excessive sedation, confusion, or even serotonergic toxicity. Strong CYP2D6 inhibitors include certain antidepressants, antipsychotic medications, and some cardiovascular drugs.
The interaction between dextromethorphan and monoamine oxidase inhibitors represents one of the most serious drug interaction concerns in cough medicine. This combination can precipitate serotonin syndrome, a potentially life-threatening condition characterized by altered mental status, autonomic dysfunction, and neuromuscular abnormalities. Patients taking monoamine oxidase inhibitors should avoid dextromethorphan-containing medications entirely, and adequate washout periods must be observed when transitioning between these medication classes.
Selective serotonin reuptake inhibitors and other serotonergic medications can also interact with dextromethorphan to increase the risk of serotonin syndrome, although the risk is generally lower than with monoamine oxidase inhibitors. Healthcare providers should be aware of this potential interaction when prescribing or recommending dextromethorphan to patients taking antidepressant medications, and patients should be counseled about the signs and symptoms of serotonergic toxicity.
Opioid antitussives present additional interaction concerns due to their central nervous system depressant effects and potential for additive toxicity when combined with other depressant medications. The combination of opioid antitussives with alcohol, benzodiazepines, or other central nervous system depressants can result in profound sedation, respiratory depression, and potentially fatal outcomes. These interactions are particularly concerning given the widespread availability of over-the-counter cough medications containing opioid ingredients.
Guaifenesin interactions are generally less clinically significant than those involving antitussive medications, but some considerations remain important for optimal therapy. The medication can potentially affect the absorption of certain other drugs when administered concurrently, and patients should be advised about appropriate timing of administration when taking multiple medications. The high fluid intake recommended with expectorant therapy may also affect the pharmacokinetics of other medications by altering gastric pH, transit time, or renal elimination.
The interaction potential of combination cough products is significantly increased compared to single-ingredient formulations due to the presence of multiple active ingredients with different pharmacological profiles. These products may contain not only cough-specific medications but also decongestants, antihistamines, and analgesics, each with their own interaction potential. Healthcare providers should carefully review all active ingredients in combination products and assess for potential interactions with existing medication regimens.
Acetylcysteine interactions include potential effects on certain antibiotics, particularly tetracyclines and some beta-lactam antibiotics, where concurrent oral administration may reduce antibiotic absorption and effectiveness. The reducing properties of acetylcysteine can also interact with certain metals and should be considered when patients are receiving other medications containing metal ions or when using medical devices containing metallic components.
The assessment of drug interaction risk requires comprehensive medication history taking that includes not only prescription medications but also over-the-counter products, dietary supplements, herbal preparations, and recreational substances. Many patients do not consider over-the-counter cough medications as “real drugs” and may fail to mention their use unless specifically questioned about these products.
Age-related changes in drug metabolism and elimination can significantly affect interaction risk in elderly patients, who may also be taking multiple medications for chronic conditions. The increased prevalence of polypharmacy in geriatric populations, combined with age-related changes in hepatic and renal function, creates a heightened risk for clinically significant drug interactions involving cough medications.
Clinical Decision-Making Algorithms for Cough Medicine Selection
The development of systematic approaches to cough medicine selection requires integration of multiple clinical factors including cough characteristics, patient demographics, underlying medical conditions, and individual risk factors. These decision-making frameworks help ensure that therapeutic choices are based on evidence-based principles while accounting for patient-specific considerations that may influence treatment outcomes.
The initial assessment of cough characteristics provides the foundation for subsequent therapeutic decisions and should include evaluation of cough duration, productivity, timing patterns, associated symptoms, and triggering factors. Acute coughs lasting less than three weeks are typically associated with viral respiratory infections and may be managed symptomatically with either antitussive or expectorant therapy depending on their productive nature. The presence of fever, purulent sputum, or systemic symptoms may indicate bacterial infection requiring antibiotic therapy in addition to symptomatic cough management.
Chronic coughs persisting beyond eight weeks require more comprehensive evaluation to identify underlying causes such as asthma, gastroesophageal reflux disease, chronic obstructive pulmonary disease, or medication-induced cough. The management of chronic cough should focus primarily on treating the underlying condition rather than relying solely on symptomatic cough suppression, although adjunctive cough medications may provide additional symptomatic relief during the diagnostic workup and initial treatment phases.
The distinction between productive and non-productive coughs represents a critical decision point in medication selection, although this distinction may not always be straightforward in clinical practice. Truly dry, non-productive coughs that serve no beneficial purpose are appropriate candidates for antitussive therapy, particularly when they interfere with sleep, daily activities, or recovery from underlying illness. However, coughs that appear dry but are associated with difficulty expectorating thick secretions may benefit more from expectorant or mucolytic therapy.
Patient age significantly influences medication selection and dosing considerations, with particular caution required in pediatric and geriatric populations. Children under four years of age generally should not receive over-the-counter cough medications due to safety concerns and limited efficacy data, while elderly patients may require dose adjustments and enhanced monitoring due to age-related changes in drug metabolism and increased susceptibility to adverse effects.
Comorbid medical conditions play a crucial role in medication selection and may contraindicate certain therapeutic approaches. Patients with chronic obstructive pulmonary disease or other conditions associated with impaired secretion clearance may not be appropriate candidates for antitussive therapy that could further impair their ability to clear respiratory secretions. Conversely, patients with congestive heart failure or other conditions where excessive coughing could be harmful may benefit from judicious use of antitussive medications.
The medication history review should identify potential drug interactions, contraindications, and previous responses to cough medications that may guide current therapeutic decisions. Patients who have previously experienced adverse effects from specific cough medications should avoid those agents, while those who have had good responses to particular medications may benefit from similar therapeutic approaches for new episodes of cough.
The severity and impact of cough symptoms on patient function and quality of life should influence the aggressiveness of therapeutic intervention and the willingness to accept potential risks associated with more potent medications. Mild coughs that do not significantly impact daily activities may be managed with conservative approaches and simple supportive measures, while severe coughs that interfere with sleep, work, or social functioning may warrant more aggressive pharmacological intervention.
Environmental and lifestyle factors may also influence medication selection and effectiveness. Patients exposed to respiratory irritants in their work or home environment may benefit from removal or reduction of these exposures in addition to pharmacological therapy. Smokers may require different therapeutic approaches due to the chronic irritant effects of tobacco smoke and the potential for impaired medication effectiveness.
| Cough Type | Primary Characteristics | Recommended Medication Class | Key Considerations |
| Dry, Non-productive | No sputum production, irritating, interferes with sleep | Antitussives (Dextromethorphan) | Avoid if secretions are present but difficult to expectorate |
| Productive with Thin Secretions | Easy expectoration, watery sputum | Supportive care, hydration | Medication may not be necessary |
| Productive with Thick Secretions | Difficult expectoration, viscous sputum | Expectorants (Guaifenesin) or Mucolytics | Ensure adequate hydration |
| Chronic Productive | Persistent thick secretions, underlying disease | Mucolytics (Acetylcysteine) | Address underlying condition |
Special Populations and Individualized Therapy Approaches
The management of cough symptoms in special populations requires careful consideration of unique physiological, pharmacological, and clinical factors that may significantly impact medication selection, dosing, and monitoring approaches. These populations include pregnant and breastfeeding women, patients with chronic kidney or liver disease, immunosuppressed individuals, and those with multiple comorbid conditions that may complicate standard therapeutic approaches.
Pregnancy presents particular challenges for cough medication management due to concerns about fetal safety and the physiological changes that occur during gestation. The safety data for most cough medications during pregnancy is limited, with few medications having been subjected to rigorous clinical trials in pregnant populations. Dextromethorphan is generally considered relatively safe during pregnancy based on observational studies and case reports, but should still be used judiciously and only when the benefits clearly outweigh potential risks.
Guaifenesin use during pregnancy has a more limited safety database, although no major teratogenic effects have been reported in available studies. The medication should be used with caution during the first trimester when organogenesis is occurring, and pregnant women should be counseled about the importance of adequate hydration when using expectorant medications. Non-pharmacological approaches such as humidification, positioning, and irritant avoidance may be preferable first-line interventions for pregnant women with mild cough symptoms.
Breastfeeding mothers require consideration of medication transfer into breast milk and potential effects on nursing infants. Dextromethorphan appears to have minimal transfer into breast milk and is generally considered compatible with breastfeeding, while the transfer characteristics of guaifenesin are less well studied. Healthcare providers should weigh the benefits of maternal symptom relief against potential risks to the nursing infant and consider timing of medication administration relative to nursing schedules.
Patients with chronic kidney disease require special attention to medication dosing and monitoring due to altered drug elimination and increased susceptibility to adverse effects. Guaifenesin, which relies partially on renal elimination, may require dose adjustments in patients with significant kidney dysfunction. The accumulation of drug metabolites in patients with impaired kidney function can lead to unexpected adverse effects even with standard dosing regimens.
Liver disease significantly affects the metabolism of many cough medications, particularly those that undergo extensive hepatic biotransformation such as dextromethorphan. Patients with cirrhosis or other forms of severe liver disease may experience prolonged and enhanced drug effects due to reduced metabolic capacity, requiring substantial dose reductions and enhanced monitoring for adverse effects. The assessment of liver function should guide dosing decisions and may contraindicate the use of certain medications in patients with severe hepatic impairment.
Immunosuppressed patients present unique challenges due to their increased susceptibility to respiratory infections and potential for atypical presentations of respiratory illness. Cough in these patients may represent serious underlying pathology that requires prompt medical evaluation rather than symptomatic treatment alone. The use of cough suppressants in immunosuppressed patients should be approached with particular caution, as suppression of the cough reflex could potentially impair the clearance of infected material from the respiratory tract.
Elderly patients represent a particularly vulnerable population due to age-related changes in pharmacokinetics and pharmacodynamics, increased prevalence of comorbid conditions, and higher risk of polypharmacy-related drug interactions. The “start low and go slow” principle is particularly relevant in geriatric populations, with initial doses often requiring reduction by 25-50% compared to standard adult dosing. Enhanced monitoring for adverse effects is essential, as elderly patients may be more susceptible to sedation, confusion, and falls associated with cough medications.
Patients with respiratory compromise from conditions such as chronic obstructive pulmonary disease, interstitial lung disease, or neuromuscular disorders require careful evaluation before initiation of cough medications. Antitussive therapy may be contraindicated in these patients if cough suppression could impair necessary secretion clearance, while expectorant therapy may be beneficial but require additional supportive measures to ensure effective secretion elimination.
The individualization of cough therapy also extends to consideration of patient preferences, lifestyle factors, and treatment goals. Some patients may prioritize rapid symptom relief even at the cost of potential side effects, while others may prefer more conservative approaches with emphasis on safety and tolerability. Healthcare providers should engage in shared decision-making that incorporates patient values and preferences into therapeutic planning.
| Special Population | Key Considerations | Preferred Medications | Monitoring Requirements |
| Pregnancy | Fetal safety concerns, physiological changes | Conservative doses of dextromethorphan | Monitor maternal symptoms and fetal well-being |
| Elderly | Altered pharmacokinetics, polypharmacy risk | Reduced doses, simple regimens | Enhanced adverse effect monitoring |
| Kidney Disease | Drug accumulation, altered elimination | Dose-adjusted guaifenesin | Monitor kidney function and drug effects |
| Liver Disease | Impaired metabolism, prolonged effects | Avoid hepatically metabolized drugs | Monitor liver function and neurological status |
Emerging Therapies and Future Directions in Cough Management
The field of cough medicine continues to evolve with advancing understanding of cough pathophysiology and the development of novel therapeutic approaches that target previously unexplored mechanisms. Recent research has identified new neurological pathways involved in cough sensation and transmission, leading to investigation of medications that can modulate these pathways for more effective and targeted cough management.
P2X3 receptor antagonists represent one of the most promising areas of current cough medication development. These receptors are found on sensory neurons in the respiratory tract and play crucial roles in detecting and transmitting cough-inducing stimuli. Several P2X3 antagonists are currently in clinical development and have shown encouraging results in early trials for patients with chronic refractory cough. These medications could potentially provide relief for patients who do not respond adequately to conventional cough medications.
Neurokinin-1 receptor antagonists, originally developed for other indications such as nausea and vomiting, have shown potential efficacy for cough suppression in preliminary studies. These medications work by blocking substance P, a neuropeptide involved in pain and cough transmission. The dual mechanism of action could provide benefits for patients with cough associated with neuropathic pain or hypersensitivity syndromes.
TRPV1 receptor modulators represent another innovative approach to cough management, targeting the vanilloid receptors that respond to irritant stimuli in the respiratory tract. These receptors are involved in detecting noxious stimuli and initiating protective reflexes, making them attractive targets for medications designed to reduce cough hypersensitivity without completely suppressing beneficial protective responses.
Gene therapy approaches for cough management are in early investigational stages but could potentially provide long-lasting therapeutic effects for patients with genetic conditions affecting respiratory secretion production or clearance. These approaches might be particularly relevant for patients with cystic fibrosis or other monogenic disorders affecting respiratory function.
Personalized medicine approaches to cough management are becoming increasingly sophisticated with the development of genetic testing for drug metabolism variants and the identification of biomarkers that predict treatment response. Pharmacogenomic testing for CYP2D6 variants could help optimize dextromethorphan dosing and identify patients at risk for adverse effects or treatment failure.
Novel drug delivery systems including targeted inhalation devices, sustained-release formulations, and combination products are being developed to improve medication effectiveness and patient convenience. These delivery systems could potentially achieve higher local drug concentrations in the respiratory tract while minimizing systemic exposure and side effects.
The integration of digital health technologies into cough management includes smartphone applications for symptom tracking, telemedicine consultations for medication adjustment, and artificial intelligence algorithms for treatment optimization. These technologies could improve patient engagement with therapy and provide healthcare providers with more detailed information about treatment response and adherence patterns.
Practical Implementation and Patient Education Strategies
The successful implementation of cough medication therapy requires comprehensive patient education that addresses not only proper medication use but also realistic expectations about treatment outcomes, potential side effects, and the importance of appropriate follow-up care. Effective patient education programs should be tailored to individual patient needs, literacy levels, and cultural backgrounds to ensure optimal understanding and compliance with therapeutic recommendations.
Patients should receive clear instructions about proper medication dosing that include not only the amount and frequency of administration but also the timing relative to meals, other medications, and daily activities. The importance of adhering to recommended dosing intervals should be emphasized, as both underdosing and overdosing can significantly impact treatment effectiveness and safety. Written instructions and dosing calendars may be helpful for patients who take multiple medications or have complex dosing regimens.
The education about expected treatment outcomes should include realistic timelines for symptom improvement and guidance about when to seek additional medical care if symptoms persist or worsen. Patients using antitussive medications should understand that complete cough suppression may not be achievable or desirable, while those using expectorant medications should be prepared for potential initial increases in cough productivity as secretions are mobilized.
Side effect education should cover both common and serious adverse effects associated with specific medications, with emphasis on symptoms that require immediate medical attention. Patients should be instructed to discontinue medication and seek medical care if they experience severe side effects such as difficulty breathing, severe dizziness, or allergic reactions. The importance of avoiding alcohol and other central nervous system depressants when using antitussive medications should be clearly communicated.
Drug interaction education should include instructions to inform all healthcare providers about cough medication use, including over-the-counter products that patients may not consider important to mention. Patients should be advised to check with pharmacists or healthcare providers before starting new medications, including prescription drugs, over-the-counter products, and dietary supplements that could potentially interact with their cough medications.
The role of non-pharmacological interventions should be emphasized as important adjuncts to medication therapy. Patients should receive education about proper hydration, humidification of inspired air, avoidance of respiratory irritants, and positioning techniques that may enhance treatment effectiveness. The importance of smoking cessation for patients who smoke should be strongly emphasized, as continued smoking can significantly impair cough medication effectiveness.
Follow-up care instructions should specify when patients should return for reassessment, what symptoms should prompt earlier medical consultation, and how to access care if problems arise outside of normal business hours. Patients with chronic cough conditions may require different follow-up schedules compared to those with acute, self-limited symptoms.
The development of medication adherence strategies should address common barriers to compliance such as side effects, complex dosing regimens, cost concerns, and lack of perceived benefit. Healthcare providers should work with patients to identify individual barriers and develop personalized strategies to improve adherence and treatment outcomes.
Patient education materials should be available in multiple formats and languages to accommodate diverse patient populations. Written materials, video resources, and interactive tools can reinforce verbal instructions and provide patients with references they can consult at home. The use of teach-back methods during patient education encounters can help ensure that patients understand key concepts and instructions.
Conclusion and Clinical Practice Integration
The optimal management of cough symptoms through appropriate medication selection represents a complex clinical challenge that requires integration of sophisticated pharmacological knowledge with individualized patient assessment and shared decision-making principles. The distinction between antitussive and expectorant approaches to cough management reflects fundamental differences in therapeutic philosophy and mechanism of action that must be carefully matched to specific patient presentations and treatment goals.
The evidence base supporting cough medication use continues to evolve, with ongoing research providing new insights into optimal therapeutic approaches and patient selection criteria. Healthcare providers must remain current with emerging evidence while maintaining critical evaluation of marketing claims and promotional materials that may not accurately represent clinical efficacy or safety data.
The future of cough medicine lies in increasingly personalized approaches that consider individual patient characteristics, genetic factors, and specific disease mechanisms to optimize therapeutic outcomes. The development of novel therapeutic targets and delivery systems promises to expand treatment options for patients who do not respond adequately to current medications or who experience unacceptable side effects with conventional therapy.
The integration of cough medication management into comprehensive respiratory care requires coordination with other therapeutic interventions and ongoing monitoring of treatment response and adverse effects. Healthcare providers should maintain systematic approaches to medication selection and adjustment that prioritize patient safety while striving to achieve optimal symptom control and functional improvement.
Patient education and shared decision-making remain central to successful cough medication therapy, ensuring that patients understand their treatment options, have realistic expectations about outcomes, and are empowered to participate actively in their care. The investment in comprehensive patient education often pays dividends in improved adherence, better outcomes, and enhanced patient satisfaction with care.
The careful application of evidence-based principles to cough medication selection, combined with individualized patient assessment and ongoing monitoring, represents the foundation of effective cough management that can significantly improve patient quality of life while minimizing risks and optimizing therapeutic outcomes. As our understanding of cough pathophysiology continues to advance and new therapeutic options become available, the principles outlined in this comprehensive analysis will continue to guide clinical decision-making and therapeutic optimization in this important area of respiratory medicine.
 sneezealert.com
sneezealert.com
